Laboratoire Riva Inc. is recalling one lot (lot C9323) of Riva-Risperidone 0.25 mg tablets due to a packaging error. Some bottles may incorrectly contain only Riva-Gabapentin 100 mg capsules. Pharmacists may not recognize the error and inadvertently repackage and dispense pill bottles that contain the wrong medication.
Health Canada is monitoring the company's recall.
Risperidone is a prescription drug used in adults to treat the symptoms of schizophrenia and related psychotic disorders, as well as bipolar disorder. It may also be used for the short-term treatment of severe dementia related to Alzheimer's disease.
Gabapentin is a prescription drug used for treating epilepsy (seizures).
If you miss a dose of Risperidone, you may not have proper control of your condition or its symptoms may worsen.
By taking Gabapentin instead of Risperidone, you may:
- develop serious side effects, such as swelling of the legs, ankles or feet (edema)
- experience side effects, such as agitation, drowsiness, dizziness, lack of muscle coordination and fatigue
If you notice any of these signs or symptoms, contact your health care professional immediately.
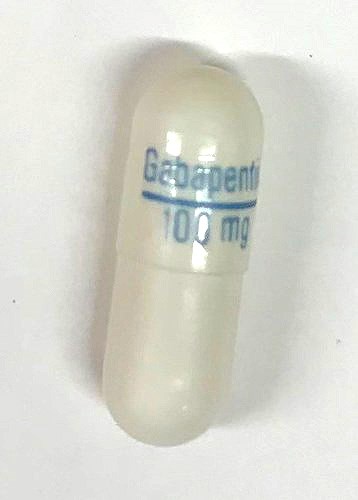
Riva-Risperidone 0.25 mg tablets are yellowish-orange, oblong-shaped coated tablets, with "0.25" on one side and "R" on the other side. Riva-Gabapentin 100 mg capsules are white hard gelatin capsules, with "Gabapentin / 100 mg" printed on the capsule in blue ink.
What you should do
- If your pill bottle of Riva-Risperidone contains white capsules, or if you are unsure, return it to your pharmacy as soon as possible for a replacement. Your pharmacist will check it and provide a replacement if needed.
- Contact your health care professional immediately if you notice that your condition or symptoms are getting worse, or if you are experiencing serious side effects.
- Contact Laboratoire Riva Inc. by calling 1-800-363-7988 or emailing [email protected] if you have questions about the recall.
- Report any health product-related side effects or complaints to Health Canada.